BfR
|
Annual Report 2014
58
Long-term effects of chemicals
To be able to draw conclusions on the potential long-
term effects of chemicals on health or in the environ-
ment, certain toxicity studies for chemicals are re-
quired in the REACH Regulation. This includes repeat-
ed dose toxicity, mutagenicity, toxicity to reproduction
and ecotoxicity. In order to evaluate the environmental
fate of a substance, studies are required on biotic
and abiotic degradability and bioaccumulation.
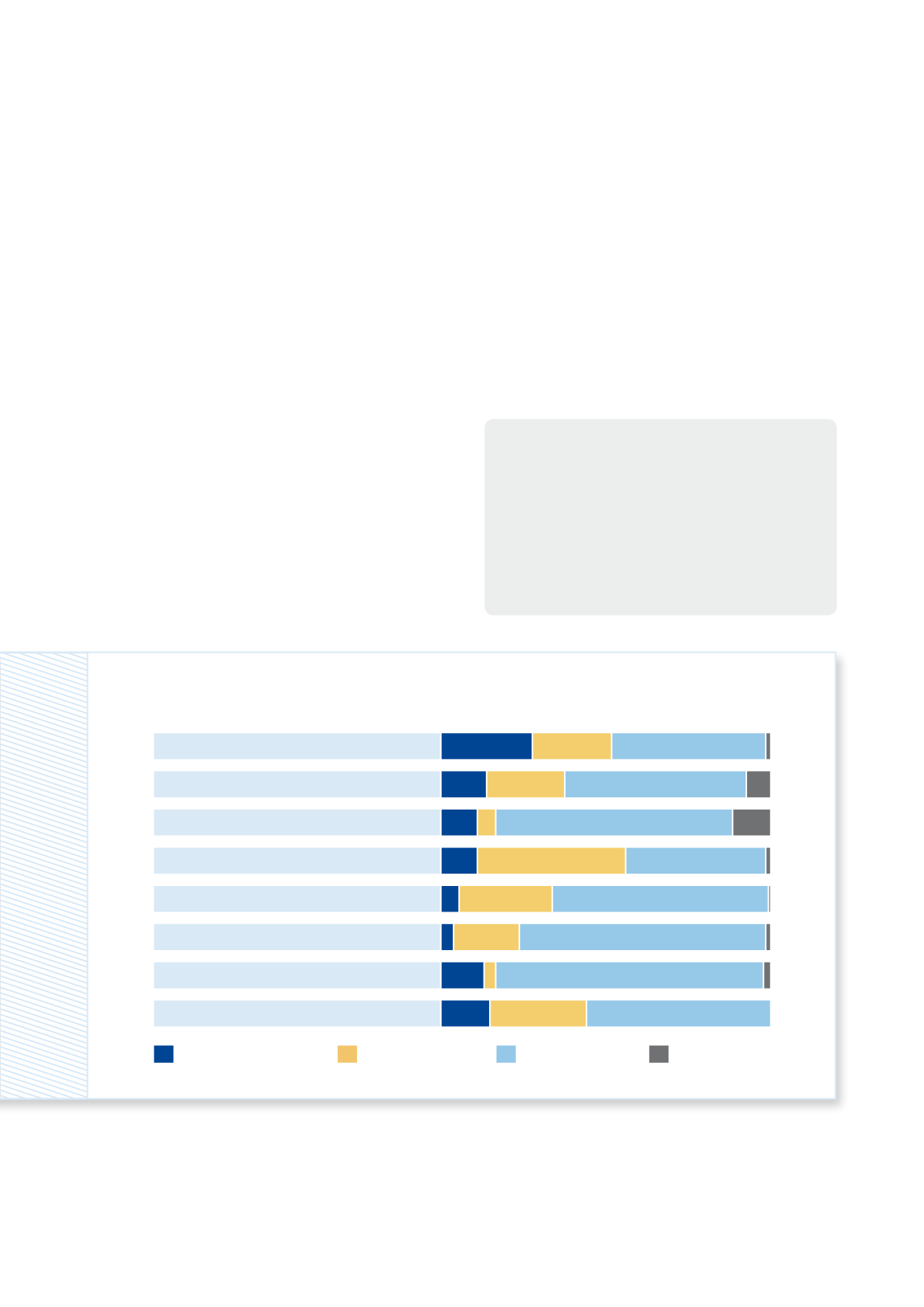
Data availability in REACH registration dossiers
The BfR reviewed 1,932 REACH registration dossiers to verify whether they contained the required information regarding
potential long-term effects of chemicals. As a result, it was judged whether the available information complies with the
REACH requirements (= category “compliant”) or not (=category “non-compliant”) or whether no conclusive decision
could be made within the scope of the project (= category “complex” and “testing proposal”).
Abiotic degradation
Bioaccumulation
Ecotoxicity
Environmental exposure
Biotic degradation
Toxicity to reproduction
Repeated dose toxicity
Mutagenicity
Non-compliant
Compliant
Complex
Testing proposal
these registration dossiers. In this project, therefore, no
conclusive decisions could be made for these cases with
regard to the conformity of the data with the standard
requirements. However, some of these issues will be now
investigated further in a follow-up project.
The BfR, the German Federal Environmental Agency
(UBA) and ECHA are using the results of the project to
identify substances which require regulation and to carry
out risk management measures. The project was carried
out in collaboration with UBA and funded by the Federal
Ministry for the Environment, Nature Conservation, Build-
ing and Nuclear Safety.
In order to systematically compare the information pro-
vided with the standard requirements set out in REACH
for high tonnage substances, decision trees were devel-
oped within the project for a wiki-based software. Us-
ing this, the BfR checked which standard requirements
were fulfilled or not fulfilled by the registration dossiers or
whether no conclusive decision could be made.
The analysis showed that information was missing in
more than half of the registration dossiers. For example,
in a quarter of the dossiers the data on mutagenicity ef-
fects of the registered substances were incomplete (see
chart). It also became clear that registrants frequently
used the legal option of deviating from the standard re-
quirements. For example, they submitted data collected
by using methods that are no longer recognised today.
Data regarding structurally similar substances were also
submitted in order to predict the effects of a registered
substance on human health and the environment.
If the legal requirements for the registered substance are
fulfilled by deviating information, then scientific justifica-
tion is required. As specific data are usually submitted for
this, case-by-case assessments must be carried out for


