57
Chemicals Safety
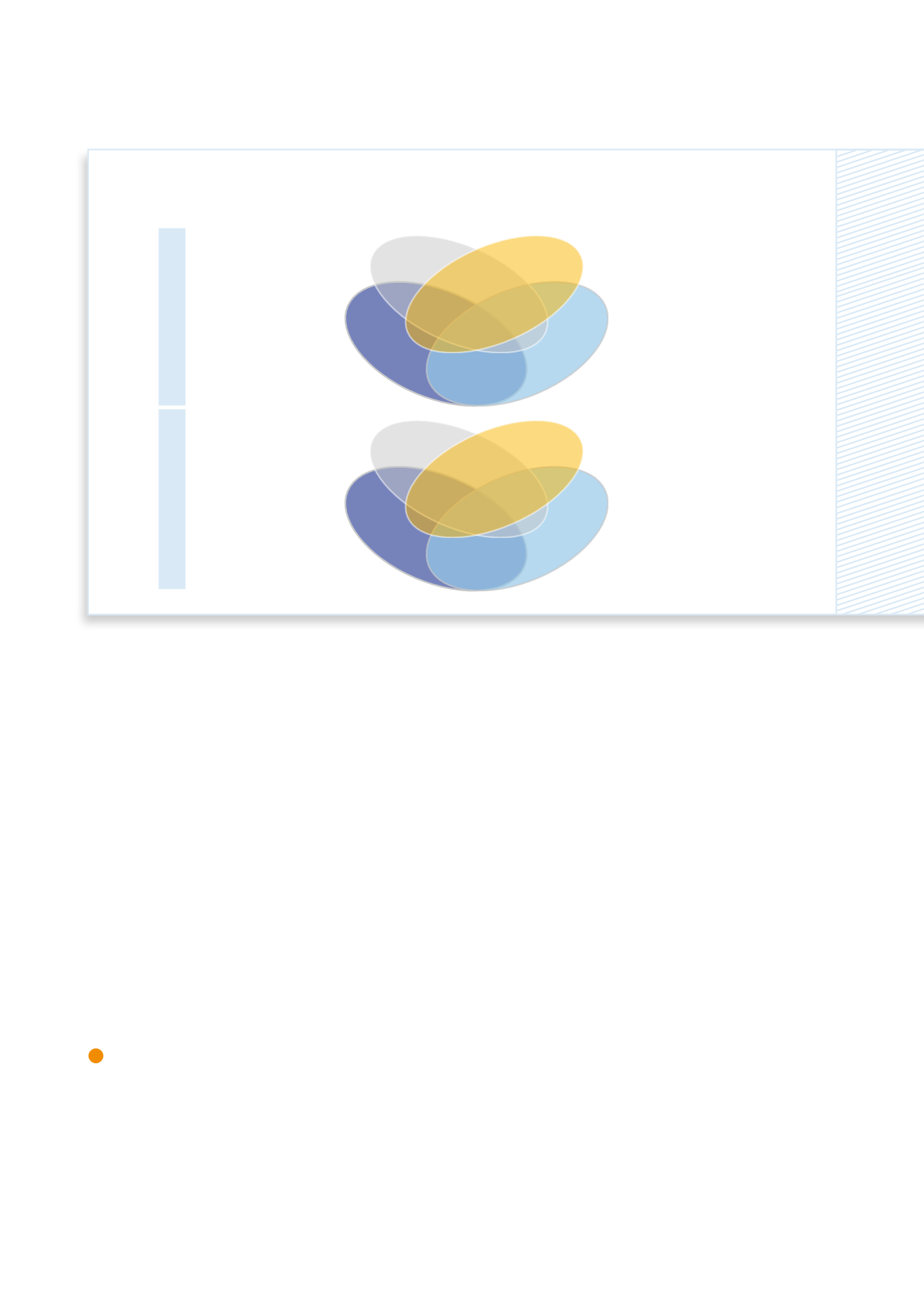
The diagram shows the number of modified genes in the animal experiment (above) and the cell culture experiment
(below) for individual substances and combinations (mixture 1).
The BfR's results on the combination effect of fungicides using omics methods
The question how results obtained by omics methods can
be used for regulatory purposes was the focus of an in-
ternational experts' workshop at the BfR in October 2014.
The following important conclusions were drawn:
>
>
Harmful effects of a chemical can currently only be
clearly determined in intact organisms. However, the
new parameters and methods can help to identify
mechanisms of action and to determine their rele-
vance for humans.
>
>
For more extensive use of omics methods in the future,
associations between the obtained data and clinical
or pathological modifications must be shown. It will
be necessary to use different methods independently
and to combine the results for a reliable conclusion. It
will not be possible to rely on an individual test.
>
>
Validation of the individual methods is a prerequisite
for their use for regulatory purposes.
Epoxiconazole
Mixture I
Mixture I
Epoxiconazole
Mixture I
Prochloraz
Cyproconazole
animal experiment
cell culture experiment
69
105
51
47
30
37
37
53
32
33
42
59
64
35
Prochloraz
Cyproconazole
18
19
7
7
2
3
4
4
2
2
6
30
8
2
Do registration dossiers of chemicals meet
the legal requirements?
In the European Union, chemicals may only be used
when the risks they pose can be adequately controlled.
To this end, they must be registered at the European
Chemicals Agency (ECHA). When registering a sub-
stance, information regarding the harmful effects of the
chemicals on humans and the environment must be pro-
vided. What information is mandatory is laid down in the
European Chemicals Regulation (REACH).
In a research project, the BfR reviewed 1,932 registration
dossiers of chemicals that are produced in particularly
large amounts – over 1,000 tonnes per year. It was veri-
fied whether the registrants provided all of the required
information on the important long-term effects of these
chemicals.


